Hypertension (high blood pressure) is one of the most common cardiovascular risk factors, and its prevalence continues to rise. According to a recent study, there are more than 1.3 billion people living with hypertension worldwide – a startling number, which has almost doubled in the past 40 years. Left uncontrolled, people have a greater risk of life-threatening conditions such as heart attack, heart failure, stroke, and chronic kidney disease.
Patients with hypertension can often successfully control their blood pressure by combining a healthier lifestyle with effective medication. However, approximately 10% of patients have resistant hypertension where the blood pressure remains high despite receiving at least three antihypertensive medications of different pharmacological classes, including a diuretic, at optimal doses.
There are more than 260 antihypertensive therapies available (single agents and fixed combinations), acting via 8 different pharmacological pathways. Most of these therapies were introduced decades ago and, although hypertension is a serious and growing problem around the world, it has been almost 40 years since an antihypertensive drug working via a new pathway was last brought to the market.
Treatment guidelines and hypertension textbooks consistently emphasize that the use of a diuretic is critically important in the treatment of resistant hypertension, as – from a pathophysiological standpoint – it is considered to be a volume-dependent hypertension. The current pharmacological treatment strategy for patients with resistant hypertension is to add on antihypertensive medications, preferably with a mechanism of action which has not yet been used.
Endothelin-1 (ET-1) is a potent vasoconstrictor that also induces neurohormonal activation, vascular hypertrophy and remodeling, cardiac hypertrophy and fibrosis, and endothelial dysfunction.
The endothelin pathway has been implicated in the pathogenesis of hypertension, especially in volume- and salt-dependent forms, which are a common feature in patients with resistant hypertension, but it is not currently targeted therapeutically, thereby leaving this relevant pathophysiological pathway unopposed with currently available medications. This pathway is activated in patients prone to developing resistant hypertension, such as aging patients, Black or African-American patients, obese patients, or those with obstructive sleep apnea, as well as in comorbid conditions frequently associated with resistant hypertension, such as diabetes and chronic kidney disease.
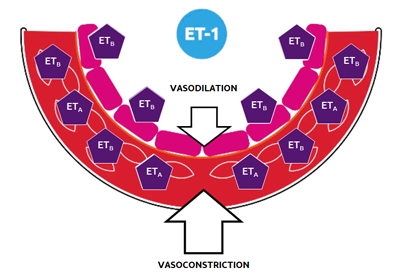
Aprocitentan is a once-daily, orally active, dual endothelin receptor antagonist, which potently inhibits the binding of ET-1 to ETA and ETB receptors. Aprocitentan has a half-life of approximately 41 hours and a low drug–drug interaction potential, which is particularly important for patients who are typically being treated for several other problems.
In a Phase 2 dose-response study, patients with hypertension received monotherapy with four doses of aprocitentan or placebo (lisinopril was used as a positive control) for eight weeks, using a randomized, double-blind study design. There was a clear dose response on both diastolic and systolic blood pressure, with clinically relevant effects observed at 10 mg, 25 mg, and 50 mg, with no additional effect at 50 mg. The effect of aprocitentan was shown to cover a 24-hour period. The overall incidence of adverse events observed in the aprocitentan groups (ranging from 22.0% to 40.2%) was similar to that seen in the placebo group (36.6%). Overall, the most common events were hypertension, headache, and nasopharyngitis.
The data from the preclinical and clinical program gave Idorsia the confidence to embark on a large registration study, PRECISION, in patients with resistant hypertension.
PRECISION was a multicenter, blinded, randomized, parallel-group, Phase 3 study, which was performed in hospitals or research centers in Europe, North America, Asia, and Australia. Patients were eligible for randomization if their sitting systolic blood pressure (SBP) was 140 mm Hg or higher despite taking standardized background therapy consisting of three antihypertensive drugs, including a diuretic. The study consisted of three sequential parts: Part 1 was the 4-week double-blind, randomized, and placebo-controlled part, in which 730 patients were randomized to aprocitentan 12.5 mg (n=243), aprocitentan 25 mg (n=243), or placebo (n=244) in a 1:1:1 ratio; Part 2 was a 32-week single (patient)-blind part, in which all patients received aprocitentan 25 mg (n=704); and Part 3 was a 12-week double-blind, randomized, and placebo-controlled withdrawal part, in which patients were re-randomized to aprocitentan 25 mg (n=307) or placebo (n=307) in a 1:1 ratio. The primary and key secondary endpoints were changes in unattended office SBP from baseline to week 4 and from withdrawal baseline to week 40, respectively. Secondary endpoints included 24-h ambulatory blood pressure changes.
At baseline, 69.2% of patients were obese or severely obese, 54.1% had diabetes, 22.2% had stage 3-4 chronic kidney disease and 19.6% had congestive heart failure. At screening, 63% of all patients who were randomly assigned were prescribed four or more antihypertensive drugs.
Detailed results were published by Schlaich MP, et al. in The Lancet and presented as a Late-Breaking Science presentation during the American Heart Association (AHA) Scientific Sessions in November 2022.
Current status
In March 2024, TRYVIO™ (aprocitentan) 12.5 mg was approved by the US FDA for the treatment of hypertension in combination with other antihypertensive drugs, to lower blood pressure in adult patients who are not adequately controlled on other drugs. For more information see the Full Prescribing Information including BOXED Warning (PI and Medication Guide). The company is preparing to make TRYVIO available during the second half of 2024.

On April 25, 2024, the Committee for Medicinal Products for Human Use (CHMP), the scientific committee of the European Medicines Agency (EMA), adopted a positive opinion for the use of JERAYGO™ (aprocitentan) for the treatment of resistant hypertension in adult patients in combination with at least three antihypertensive medicinal products. The CHMP has adopted a positive opinion for the use of 12.5 mg JERAYGO orally once daily. The dose can be increased to 25 mg once daily for patients tolerating the 12.5 mg dose and in need of tighter blood pressure control.
Detailed recommendations for the use of JERAYGO will be described in the summary of product characteristics (SmPC), which will be published in the European public assessment report (EPAR) and made available in all official European Union languages after the marketing authorization has been granted by the European Commission, expected in approximately two months.
European Commission marketing authorization through the centralized procedure is valid in all European Union Member States, as well as the European Economic Area countries Iceland, Liechtenstein and Norway, and Northern Ireland under the Northern Ireland Protocol.

Milestones
2024 Positive opinion from European Union’s CHMP for JERAYGO (aprocitentan)
2024 Approved as TRYVIO (aprocitentan) by the US FDA
2023 MAA submitted to the EMA
2022 NDA submitted to the US FDA
2022 Phase 3 data simultaneously presented as late-breaker at AHA and published
in The Lancet
2022 Phase 3 study successful
2018 Phase 3 study initiated
2017 Positive results for the dose-response study
2015 Initiation of Phase 2 dose-response study
2014 Initiation of Phase 1 clinical program
Key scientific literature
- Schlaich M, et al. The Lancet 2022; Dec 3;400(10367):1927-1937.
- Iglarz M, et al. Clin Sci 2010; 119:453-63
- Clozel M. Can J Physiol Pharmacol 2022, 100:573-83.
- Verweij P., et al. Hypertension. 2020; 75:956–965
- Danaietash P et al. J Clin Hypertension 2022; 24(7):804-813