Chronic insomnia disorder is a condition of overactive wake signaling, which can have a profound effect on patients’ lives. It can be defined as a combination of dissatisfaction with sleep quantity or quality and a significant negative impact on daytime functioning. It involves difficulty initiating and/or maintaining sleep at least three times a week for a minimum of three months.
Chronic insomnia disorder as a persistent disorder is quite different from a brief period of poor sleep, and it can take its toll on both physical and mental health. Idorsia’s research has shown that poor-quality sleep can affect many aspects of daily life, including the ability to concentrate, mood, and energy levels.
Chronic insomnia disorder is a common problem, with the prevalence being approximately 10%. On this basis, and assuming a US adult population of around 250 million, there are approximately 25 million adults in the US who suffer from chronic insomnia disorder.

The goal of treatments for insomnia is to improve sleep quality and quantity, as well as daytime functioning, while avoiding next-morning residual effects. Current recommended treatment of insomnia includes sleep hygiene recommendations, cognitive behavioral therapy, and pharmacotherapy.
With regard to prescription medications, patients are treated with products indicated for insomnia, as well as off-label treatments. The on-label treatment category primarily comprises drugs that induce sleep by enhancing GABA, the primary inhibitory neurotransmitter in the brain, which works by slowing brain activity in a non-targeted manner. There are two main categories of GABA agonists – benzodiazepines and non-benzodiazepines. In addition, other approved insomnia medications include a melatonin receptor agonist and a low-dose tricyclic antidepressant.
The first products in a new class of dual orexin receptor antagonists are available in North America and certain Asia-Pacific markets. These have now been joined by daridorexant, which is available in the US and the first countries in Europe. The most widely used off-label treatment for insomnia in the US is a selective serotonin reuptake inhibitor (SSRI) which has an off-target sedation effect.
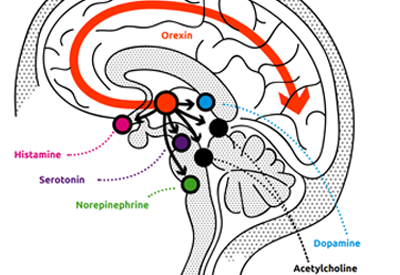
Wake and sleep signaling is regulated by intricate neural circuitry in the brain. One key component of this process is the orexin system, which helps promote wakefulness.
Orexin neuropeptides (small protein-like molecules used by nerve cells to communicate with each other in the brain) promote wakefulness through the orexin receptors OX1R and OX2R. Together, these neuropeptides and receptors make up the orexin system. The orexin system stimulates targeted neurons in the wake system, leading to the release of several chemicals (dopamine, serotonin, histamine, acetylcholine, norepinephrine) which promote wakefulness. Under normal circumstances, orexin levels rise throughout the day as wakefulness is promoted and then fall at night. Research suggests that, in chronic insomnia disorder, wake-promoting regions of the brain remain overactive at night (hyperarousal). Orexin therefore provides a specific target for pharmacotherapeutic intervention.
Dual orexin receptor antagonists (DORAs) offer an entirely different approach to treating insomnia than previous drug classes: by selectively blocking the activity of orexin, they turn down overactive wakefulness, in contrast to insomnia treatments which act via general CNS sedation. Blocking orexin receptors reduces the downstream activity of the wake-promoting neurotransmitters that are overactive in insomnia. As a result, orexin receptor antagonism targets the fundamental mechanism of insomnia.
Idorsia’s research team has been working on the science of orexin and orexin receptors since they were first described in 1998. The team’s initial work led to the conclusion that antagonism of the orexin system was the key to preserving a natural sleep architecture for patients with insomnia. With this as the target, the team designed a dual antagonist with the goal of a rapid onset of effect and a duration of action sufficient to cover the night but short enough to avoid any negative next-morning residual activity at optimally effective doses. This task proved to be very challenging, and the team synthesized more than 25,000 compounds to arrive at daridorexant.
The Phase 3 registration program comprised two three-month studies, together with a long term double-blind extension study. The program is now complete, having enrolled around 1,850 patients with insomnia. As insomnia often presents later in life, and elderly patients are more likely to experience fragmented sleep, early awakening, and daytime sleepiness, around 40% of the recruited population was aged 65 years or older.
The placebo-controlled studies investigated the effects of three doses of daridorexant (Study 1: 50 mg and 25 mg; Study 2: 25 mg and 10 mg) on sleep and daytime functioning parameters – objectively in a sleep lab by polysomnography and subjectively with a daily patient diary at home. The impact of insomnia on patients’ daytime functioning was measured daily using the sleepiness domain score from the Insomnia Daytime Symptoms and Impacts Questionnaire (IDSIQ) – a patient reported outcome (PRO) instrument.
More than 800 patients continued treatment in the 40-week extension study, which measured the effect of all three doses versus placebo, generating data for long-term treatment of insomnia.
The results of the study were reported by Mignot E, et al. in the February 2022 issue of The Lancet Neurology and have resulted in daridorexant being granted market authorrization in several countries.
Current status
In January 2022, QUVIVIQ (daridorexant) 25 mg and 50 mg was approved by the US FDA for the treatment of adult patients with insomnia, characterized by difficulties with sleep onset and/or sleep maintenance. QUVIVIQ was launched in the US in May 2022. For more information about QUVIVIQ in the US, see the Full Prescribing Information and the Medication Guide.

In April 2022, marketing authorization for QUVIVIQ was granted by the European Commission and subsequently by the Medicines and Healthcare products Regulatory Agency (MHRA) in Great Britain for the treatment of adult patients with insomnia characterized by symptoms present for at least three months and considerable impact on daytime functioning, making it Europe’s first approved dual orexin receptor antagonist.
In November 2022, QUVIVIQ was launched in Italy and Germany, followed by Spain in September 2023, the UK in October 2023, Austria in February 2024, France in March 2024, Sweden in September 2024, and Finland in June 2025. For more information about QUVIVIQ in the EU, see the Summary of Product Characteristics.
Marketing authorization for QUVIVIQ was also granted by Swissmedic in December 2022, and the company made QUVIVIQ available to patients in Switzerland in June 2023. For more information about QUVIVIQ in Switzerland, see the Patient Information and Information for Healthcare Professionals.
Market authorization for QUVIVIQ was also granted by Health Canada in April 2023, and the company made it available to patients in Canada in November 2023. For more information about QUVIVIQ in Canada, see the Product Monograph.

Idorsia is conducting a Phase 2, dose-finding study to assess the efficacy, safety, and pharmacokinetics of multiple-dose oral administration of daridorexant in pediatric patients aged 10 to <18 years with insomnia disorder (NCT05423717). The primary objective of the study is to characterize the dose-response relationship of daridorexant on objective total sleep time (TST), using polysomnography. Patients will be randomized in a 1:1:1:1 ratio to 10 mg, 25 mg, or 50 mg daridorexant, or placebo and treated for 2 weeks. The study is expected to complete enrollment by the end of 2025 with readout expected around mid-2026. The study is part of a US FDA-approved Pediatric Study Plan and an EU PDCO-approved Paediatric Investigational Plan.
Milestones
2025 Simcere received approval for QUVIVIQ in China
2025 QUVIVIQ launched in Finland
2024 Nxera launches QUVIVIQ in Japan
2024 QUVIVIQ launched in Sweden
2024 Positive results in patients with insomnia and comorbid nocturia
2024 QUVIVIQ launched in Austria and France
2023 QUVIVIQ launched in Switzerland, Spain, UK, and Canada
2023 Citizens petition filed in the US to deschedule DORA class
2023 Health Canada approves QUVIVIQ
2022 QUVIVIQ launched in Italy and Germany
2022 European Commission approves QUVIVIQ
2022 QUVIVIQ launched in the US
2022 Phase 3 data reported in The Lancet Neurology
2020 Both pivotal studies report positive results
2018 Initiation of Phase 3 registration program
2017 Completion of Phase 2 clinical program
2015 Initiation of Phase 1 clinical program
Key scientific literature
- Dauvilliers Y et al. Sleep Medicine. 2025. doi: 10.1016/j.sleep.2025.106523.
- Lederer K et al. Journal of Sleep Research. 2025. doi: 10.1111/jsr.70002.
- Fietze I., et al. 2022 Oct;39(10):795-810.
- Kunz, D. et al. CNS Drugs (2022).
- Mignot E, et al. Lancet Neurol. 2022; 21: 125–39.
- Dauvilliers, Y., et al. (2020). Ann Neurol 87(3): 347-356.
- Zammit, G., et al. (2020). Neurology 94(21): 1-11.
- Muehlan, C., et al. (2020). J Clin Psychopharmacol 40(2): 157-166.
- Muehlan, C., et al. (2020). J Psychopharmacol 34(3): 326-335.
- Boof, M. L., et al. (2019). Eur J Clin Pharmacol 75(2): 195-205.
- Muehlan, C., et al. (2019). Curr Drug Metab 20(4): 254-265.
- Muehlan, C., et al. (2019). Eur Neuropsychopharmacol 29(7): 847-857.
- Muehlan, C., et al. (2018). Clin Pharmacol Ther 104(5): 1022-1029.
- Treiber, A., et al. (2017). J Pharmacol Exp Ther 362(3): 489-503.
- Brisbare-Roch, C., et al. (2007). Nat Med 13(2): 150-5.