Fabry disease is a rare genetic, lysosomal storage disorder. It is caused by mutations in the GLA gene, leading to a deficiency or dysfunction of alpha-galactosidase A (alpha Gal A), an enzyme that normally breaks down a fatty substance known as globotriaosylceramide (Gb3) in the cells of the body. Over time, this results in an accumulation of Gb3 deposits throughout the body, leading to progressive pathophysiology in the cardiovascular system, the nervous system, and organs including the kidneys, heart, skin, ears, and eyes.
Fabry disease affects a patient’s life expectancy and quality of life. Since most symptoms are non-specific, Fabry disease is often undetected or misdiagnosed. As the disease is progressive, early diagnosis is essential to manage the symptoms as soon as possible and reduce the risk of developing serious complications.
New therapeutic options are needed to treat the underlying mechanism of the disease and provide symptomatic relief.
The prevalence of diagnosed Fabry disease in 2018 was approximately 7,500 patients in the US and the EU-5 (i.e. France, Germany, Italy, Spain, and the UK).
Clinical manifestations of Fabry disease
- Usually more severe in men
- Gradually progressing in severity from childhood to adulthood
- Major impact on quality of life
- Slow progressive damage to vital organs over decades
- Premature death

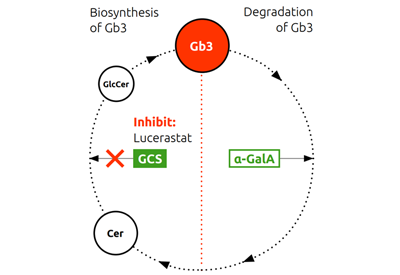
The normal biosynthesis and degradation of Gb3 is shown schematically in the Figure below. In patients with Fabry disease, deficiency or dysfunction of the enzyme alpha Gal A leads to abnormal accumulation of Gb3, which in turn causes the symptoms of Fabry disease. Current treatments focus on replacing or supporting alpha Gal A – either through infusion of recombinant enzyme, which temporarily increases plasma concentrations of alpha Gal A, or by chaperone therapy, which improves the function of mutated enzymes – but only in patients with amenable mutations.
In contrast, lucerastat, an oral inhibitor of glucosylceramide synthase (GCS), reduces the substrate which forms Gb3. Substrate reduction therapy (SRT) decreases the build-up and is thought to subsequently reduce the Gb3 load in patients with Fabry disease. Since this mechanism is independent of alpha Gal A deficiency or dysfunction, it should not be limited to specific mutations of the GLA gene.
The Gb3 cycle

Abbreviations: α-GalA, α-galactosidase A; Cer, ceramide; Gb3, globotriaosylceramide; GCS, glucosylceramide synthase; GlcCer, glucosylceramide; Sph, sphingosine
Lucerastat is an oral inhibitor of glucosylceramide synthase, offering a potential new treatment approach for all patients living with Fabry disease, irrespective of the mutation type of the GLA gene.
Preclinical studies have shown that lucerastat is an orally available, highly soluble small molecule with rapid and complete absorption. As a small molecule, it is widely distributed to most tissues, including the central nervous system, kidney, and heart.
In an animal model of Fabry disease, treatment with lucerastat reduced Gb3 levels and related biomarkers in dorsal root ganglia, the kidneys, and the heart. This demonstrates that lucerastat has the potential to reduce Gb3 levels in key target organs and, therefore, to show clinical efficacy in Fabry disease.
In an exploratory study in patients with Fabry disease, treatment with lucerastat in addition to enzyme replacement therapy induced a marked decrease in plasma levels of metabolic substrates associated with the development of the disease. The study also indicated that lucerastat is well tolerated in patients with Fabry disease.
Current status
In October 2021, the company reported that lucerastat 1000 mg b.i.d. did not meet the primary endpoint of reducing neuropathic pain during 6 months of treatment versus placebo. However, lucerastat demonstrated a substantial reduction in levels of the Fabry disease biomarker plasma Gb3 during the treatment period, with a decrease of approximately 50% observed in plasma Gb3 in the lucerastat treatment group compared to an increase of 12% in the placebo group. Lucerastat was well tolerated.
Patients continued treatment in an open-label extension (OLE) of the Phase 3 study where participants have received lucerastat for at least 42 months. The most recent interim analysis corroborated the long-term effect on plasma Gb3 levels and a potential positive long-term effect on kidney function. It also confirmed the safety and tolerability profile observed during the 6-month randomized treatment period.
Idorsia has also conducted a kidney biopsy substudy belonging to the OLE of the Phase 3 study. This substudy enrolled male participants with classic Fabry disease who had been treated for more than 3 years with lucerastat monotherapy. The main objective of the sub-study was to evaluate the number of globotriaosylceramide (Gb3) inclusions in certain types of kidney cells using established methods of quantification.
The data collected are supportive of further investigation for patients with Fabry disease and have been instrumental in the design of a new Phase 3 program. As the next steps for lucerastat are being planned, the OLE study will be concluded. To ensure continuity of care for participants still receiving lucerastat at study closure, a post-trial access program is being established. The company is collaborating with the US FDA to agree on the optimal regulatory pathway to approval.
Milestones
2025 New data and evaluation of long-term treatment with lucerastat supportive
further investigation
2021 Phase 3 open label extension study continues
2021 Phase 3 study completed – primary endpoint not met
2018 Phase 3 study initiated
2016 Phase 1b study completed
Key scientific literature
- Guérard N., et al. Clin Pharmacol Ther. 2018; 103(4):703-11.
- Welford RWD., et al. Hum Mol Genet 2018; 27(19): 3392-3403.